
Place the prepared gel upside-down on the platform.Handle membranes carefully by the edges or using clean blunt-ended forceps. Tip: Always wear gloves when working with blotting membranes. Cut one sheet of blotting membrane and two sheets of Whatman 3MM paper about 1 mm larger than the gel on each side.Remove any air bubbles between the paper and the support by rolling a pipet several times back and forth over the surface. Wet the sheets briefly in 10x SSC, and place them on the glass plate. Cut two lengths of Whatman 3MM paper wider than the gel, long enough to fit under the gel and reach to the bottom of the dish on either side.Place a support larger than the gel in a tray containing 10x SSC (see table 20x SSC), and cover the support with a glass or Plexiglas plate (see figure Southern blot setup).Incubate for 30 minutes with gentle shaking Remove the denaturation buffer and completely cover the gel in neutralization buffer (see table Neutralization buffer). If acid depurination was used to denature the DNA, the bromophenol blue will return to its original color during this incubation. Completely cover the gel with denaturation buffer (see table Denaturation buffer) and incubate for 30 minutes with gentle shaking. UV irradiation - expose the gel to UV light at a wavelength of 254 nm from a source operating at 30 W, for 30–60 seconds.ĭouble-stranded DNA must be denatured in order to create suitable hybridization targets. Depurination is therefore recommended only when fragments larger than 10 kb are to be transferred. Tip: Depurinated gels may yield “fuzzy” bands on the final autoradiograph, presumably because of increased diffusion of the DNA during transfer. Tip: The depurination step should not last too long, since very short fragments attach less firmly to the membrane. Rinse the gel briefly in distilled water. During this period the color of the bromophenol blue in the samples will change from blue to yellow, indicating that the gel has been completely saturated with the acid. In order to facilitate their transfer, these fragments are reduced in size, either by acid depurination or by UV irradiation.Īcid depurination - immediately after gel electrophoresis, place the gel in a solution of 0.2 M HCl, so that it is completely covered. Preparation of gels for Southern blottingįragmentation of large DNA molecules (optional)ĭNA fragments longer than 10 kb do not transfer to blotting membranes efficiently.Support (to be placed in the buffer tray).Buffer tray (e.g., glass casserole dish) capable of holding 1–2 liters of buffer.Paper towels, a stack of approximately 15–20 cm.A standard protocol is described here together with recipes for buffers and solutions. Many variations on the Southern blotting procedure exist. The DNA of interest can be identified by hybridization to radioactive or chemiluminescent probes and visualized by autoradiography or staining. Southern) refers to the transfer of the DNA to a nylon or nitrocellulose membrane by capillary transfer. Southern blotting (named after its inventor, E.M. The DNA is next run through an agarose gel (6). DNA is usually first converted into conveniently sized fragments by restriction digestion.
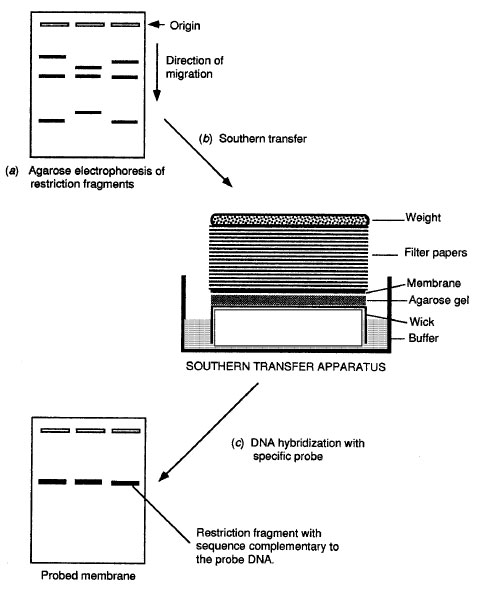
Southern blotting is a widely used technique that allows analysis of specific DNA sequences.


 0 kommentar(er)
0 kommentar(er)
